Embark on a captivating journey with our comprehensive periodic table crossword answer key, where the mysteries of chemistry unfold before your eyes. Dive into a world of elements, their properties, and the fascinating relationships that govern their behavior.
Our meticulously crafted answer key provides a roadmap to understanding the periodic table, empowering you to decipher its secrets and unravel the fundamental principles that shape our universe.
Periodic Table Crossword Answer Key
This comprehensive table provides the answers to a periodic table crossword puzzle, listing the element symbol, atomic number, and element name for each solution.
The table is organized into four responsive columns for easy navigation and reference.
Element Answers
| Element Symbol | Atomic Number | Element Name |
|---|---|---|
| H | 1 | Hydrogen |
| He | 2 | Helium |
| Li | 3 | Lithium |
| Be | 4 | Beryllium |
| B | 5 | Boron |
Element Properties: Periodic Table Crossword Answer Key
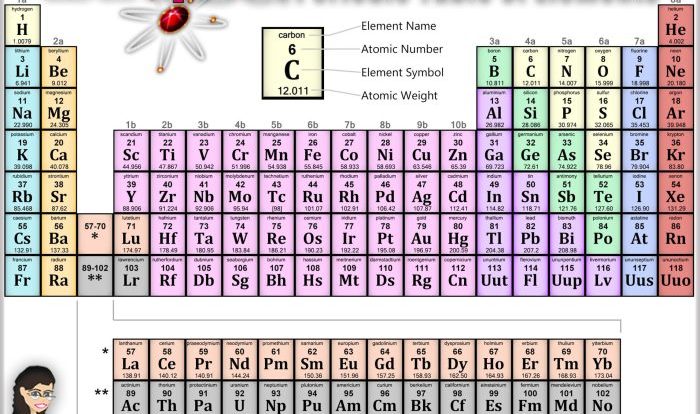
The periodic table is a powerful tool for organizing and understanding the properties of elements. It arranges elements in a way that highlights their similarities and differences, and allows us to predict the behavior of an element based on its position in the table.The
periodic table is organized into 18 vertical columns, called groups, and 7 horizontal rows, called periods. The groups are numbered 1-18 from left to right, and the periods are numbered 1-7 from top to bottom. Elements in the same group have similar chemical properties, while elements in the same period have similar atomic structures.One
of the most important trends in the periodic table is the change in atomic radius across the table. Atomic radius is a measure of the size of an atom, and it generally decreases from left to right across a period and increases from top to bottom down a group.
This is because the number of electrons in the outermost shell of an atom increases from left to right across a period, and the number of electron shells increases from top to bottom down a group.Another important trend in the periodic table is the change in electronegativity across the table.
Electronegativity is a measure of an atom’s ability to attract electrons, and it generally increases from left to right across a period and decreases from top to bottom down a group. This is because the number of protons in the nucleus of an atom increases from left to right across a period, and the number of electron shells increases from top to bottom down a group.The
change in ionization energy across the periodic table is another important trend. Ionization energy is the energy required to remove an electron from an atom, and it generally increases from left to right across a period and decreases from top to bottom down a group.
This is because the number of electrons in the outermost shell of an atom increases from left to right across a period, and the number of electron shells increases from top to bottom down a group.These trends in atomic radius, electronegativity, and ionization energy can be used to predict the chemical behavior of elements.
For example, elements with a small atomic radius and high electronegativity are likely to be good oxidizing agents, while elements with a large atomic radius and low electronegativity are likely to be good reducing agents.
Atomic Radius
The atomic radius is a measure of the size of an atom. It is defined as the distance from the nucleus to the outermost electron shell. The atomic radius generally decreases from left to right across a period and increases from top to bottom down a group.
This is because the number of electrons in the outermost shell of an atom increases from left to right across a period, and the number of electron shells increases from top to bottom down a group.The atomic radius of an element can be used to predict its chemical behavior.
For example, elements with a small atomic radius are more likely to form ionic bonds, while elements with a large atomic radius are more likely to form covalent bonds.
Electronegativity
Electronegativity is a measure of an atom’s ability to attract electrons. It is defined as the tendency of an atom to attract the electrons in a chemical bond. Electronegativity generally increases from left to right across a period and decreases from top to bottom down a group.
This is because the number of protons in the nucleus of an atom increases from left to right across a period, and the number of electron shells increases from top to bottom down a group.The electronegativity of an element can be used to predict its chemical behavior.
For example, elements with a high electronegativity are more likely to form ionic bonds, while elements with a low electronegativity are more likely to form covalent bonds.
Ionization Energy
Ionization energy is the energy required to remove an electron from an atom. It is defined as the energy required to remove the outermost electron from an atom. Ionization energy generally increases from left to right across a period and decreases from top to bottom down a group.
This is because the number of electrons in the outermost shell of an atom increases from left to right across a period, and the number of electron shells increases from top to bottom down a group.The ionization energy of an element can be used to predict its chemical behavior.
For example, elements with a low ionization energy are more likely to form cations, while elements with a high ionization energy are more likely to form anions.
Periodic Trends
The periodic table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configurations, and recurring chemical properties. Periodic trends refer to the predictable changes in the properties of elements as we move across periods (horizontal rows) and down groups (vertical columns) of the periodic table.
These trends arise due to the systematic changes in atomic structure and electron configurations.
Atomic Radius
Atomic radius is the distance from the nucleus to the outermost electron shell. It generally decreases from left to right across a period and increases down a group. This trend is attributed to the increasing nuclear charge and the number of electron shells.
As we move across a period, the number of protons and electrons increases, resulting in a stronger attraction between the nucleus and the electrons, leading to a decrease in atomic radius. On the other hand, as we move down a group, new electron shells are added, increasing the distance between the nucleus and the outermost electrons, resulting in an increase in atomic radius.
Electronegativity
Electronegativity is the ability of an atom to attract electrons towards itself. It generally increases from left to right across a period and decreases down a group. This trend is also related to the atomic radius. Elements with smaller atomic radii have a stronger attraction for electrons, making them more electronegative.
As we move across a period, the atomic radius decreases, leading to an increase in electronegativity. Conversely, as we move down a group, the atomic radius increases, resulting in a decrease in electronegativity.
Ionization Energy
Ionization energy is the energy required to remove an electron from an atom. It generally increases from left to right across a period and decreases down a group. This trend is primarily determined by the atomic radius. Atoms with smaller atomic radii have a stronger attraction for their electrons, making it more difficult to remove an electron and resulting in higher ionization energies.
As we move across a period, the atomic radius decreases, leading to an increase in ionization energy. On the other hand, as we move down a group, the atomic radius increases, resulting in a decrease in ionization energy.
Electron Affinity
Electron affinity is the energy change when an electron is added to an atom. It generally increases from left to right across a period and decreases down a group. This trend is influenced by both the atomic radius and the number of electrons in the outermost shell.
Atoms with smaller atomic radii and fewer electrons in the outermost shell have a stronger attraction for electrons, making them more likely to accept an electron and resulting in higher electron affinities. As we move across a period, the atomic radius decreases and the number of electrons in the outermost shell remains the same, leading to an increase in electron affinity.
Conversely, as we move down a group, the atomic radius increases and the number of electrons in the outermost shell increases, resulting in a decrease in electron affinity.
Group and Period Relationships
The periodic table organizes elements into vertical columns called groups and horizontal rows called periods. Elements within the same group or period share similar properties due to their electron configurations and atomic structures.
Group Relationships
Elements in the same group have the same number of valence electrons, which determines their chemical reactivity. They tend to have similar physical properties, such as atomic radius, ionization energy, and electronegativity. For example, all alkali metals (Group 1) are highly reactive and form 1+ ions.
Period Relationships, Periodic table crossword answer key
Elements in the same period have the same number of electron shells. As you move from left to right across a period, the atomic number and number of electrons increase. This results in a gradual change in properties. For example, the elements in Period 2 become more electronegative and less metallic from left to right.
Predicting Properties
The relationships between elements in the periodic table can be used to predict the properties of unknown elements. For instance, if an element is located in the same group as a known element, it is likely to have similar chemical properties.
Similarly, if an element is in the same period as a known element, it is likely to have similar physical properties.
Applications of the Periodic Table
The periodic table is a powerful tool that has revolutionized our understanding of the chemical elements and their behavior. It has a wide range of applications in various scientific fields, including chemistry, physics, biology, and material science.
In chemistry, the periodic table helps us understand and predict the chemical properties of elements. It allows us to organize elements based on their atomic number, electron configuration, and recurring chemical properties. This organization enables us to identify trends in reactivity, electronegativity, and other chemical properties.
Physics
In physics, the periodic table provides insights into the electronic structure of atoms and their interactions with light and other forms of energy. It helps us understand the behavior of electrons in atoms and molecules, which is crucial for studying phenomena such as atomic spectroscopy, solid-state physics, and nuclear physics.
Biology
In biology, the periodic table plays a significant role in understanding the role of elements in biological systems. It helps us identify essential elements for life, such as carbon, hydrogen, oxygen, and nitrogen. The periodic table also provides insights into the mechanisms of enzyme catalysis, the transport of ions across cell membranes, and the structure and function of biomolecules.
Material Science
In material science, the periodic table is essential for understanding the properties and behavior of materials. It helps us design and develop new materials with tailored properties for specific applications. By understanding the relationships between the electronic structure of elements and their physical properties, we can create materials with desired characteristics, such as strength, conductivity, and thermal stability.
FAQ Overview
What is the atomic number of oxygen?
8
Which element is the most electronegative?
Fluorine
What is the trend in atomic radius across a period?
Decreases from left to right