Determine the ph of a 0.010 m hno3 solution – Determining the pH of a 0.010 M HNO3 solution is a fundamental task in chemistry, providing insights into the acidity of the solution and its potential applications. This guide delves into the concept of pH, the dissociation of HNO3, and the methods for calculating and measuring pH, offering a comprehensive understanding of this important parameter.
Introduction

pH is a crucial concept in chemistry, representing the acidity or alkalinity of a solution. It plays a significant role in various chemical processes, industrial applications, and biological systems. Nitric acid (HNO 3) is a strong acid commonly used in laboratories and industries.
Understanding the pH of HNO 3solutions is essential for its safe handling and effective use.
Chemical Equation and Dissociation
When HNO 3dissolves in water, it undergoes dissociation, releasing hydrogen ions (H +) and nitrate ions (NO 3–):
HNO3+ H 2O → H ++ NO 3–
HNO 3is a strong acid, meaning it dissociates completely in water, releasing all of its hydrogen ions. This results in a high concentration of H +ions, making HNO 3solutions highly acidic.
pH Calculation: Determine The Ph Of A 0.010 M Hno3 Solution
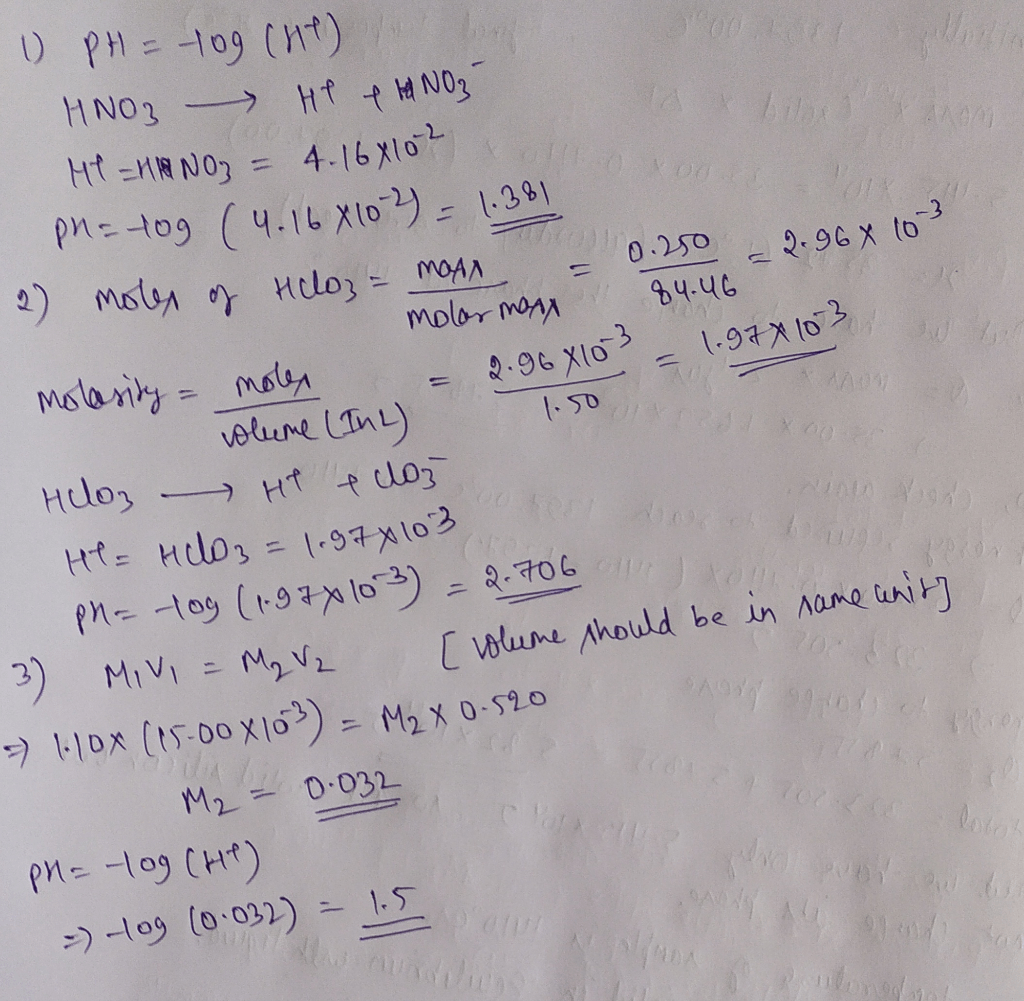
pH is calculated using the following formula:
pH =
log[H+]
where [H +] is the molar concentration of hydrogen ions in the solution. For a 0.010 M HNO 3solution, the pH can be calculated as:
pH =
log(0.010) = 2
pH Measurement
pH can be measured using various methods, including pH meters and pH paper. pH meters provide accurate and precise measurements by directly measuring the electrical potential of the solution. pH paper is a convenient and inexpensive method, but it offers less precision and is only suitable for approximate measurements.
Applications of pH
pH plays a crucial role in numerous applications, such as:
- Industrial processes:pH control is essential in various industries, including chemical manufacturing, food processing, and water treatment.
- Environmental monitoring:pH is a key indicator of water quality and can be used to assess the health of aquatic ecosystems.
- Biological systems:pH is critical for maintaining optimal conditions in biological systems, such as enzyme activity, protein structure, and cell viability.
Helpful Answers
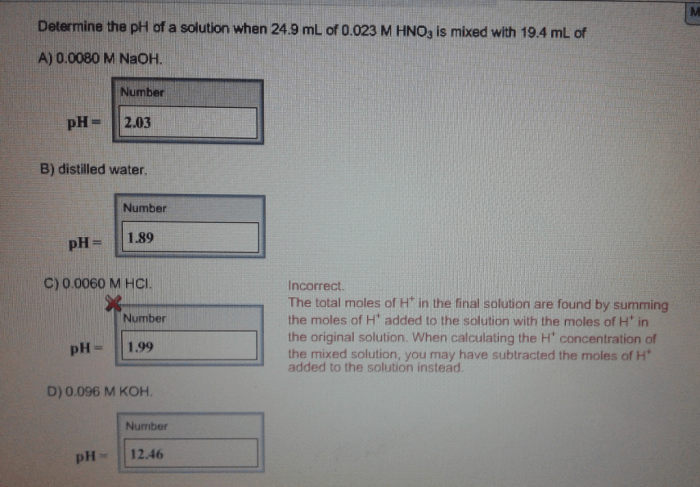
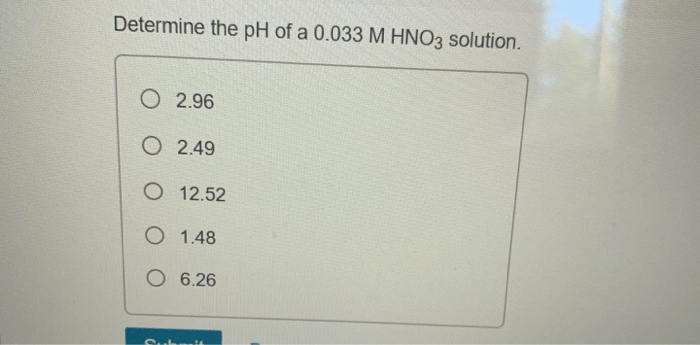
What is the pH of a 0.010 M HNO3 solution?
The pH of a 0.010 M HNO3 solution is approximately 2.00, indicating a strongly acidic solution.
Why is it important to measure pH?
pH is a crucial parameter in various fields, including chemistry, biology, and environmental science, as it affects chemical reactions, enzyme activity, and ecosystem health.
What are the different methods for measuring pH?
Common methods for measuring pH include pH meters, pH paper, and colorimetric indicators, each with its own advantages and limitations.